| Identification |
| Name: | Nitric acid, cobalt(2+)salt, hydrate (2:1:6) |
| Synonyms: | Nitricacid, cobalt(2+) salt, hexahydrate (8CI,9CI); Cobalt dinitrate hexahydrate;Cobalt nitrate (Co(NO3)2), hexahydrate; Cobalt nitrate (Co(NO3)2.6H2O); Cobaltnitrate hexahydrate; Cobalt(2+) dinitrate hexahydrate; Cobalt(2+) nitratehexahydrate; Cobalt(II) nitrate hexahydrate; Cobaltous dinitrate hexahydrate;Cobaltous nitrate hexahydrate |
| CAS: | 10026-22-9 |
| EINECS: | 233-402-1 |
| Molecular Formula: | Co. 6 H2 O . 2 H N O3 |
| Molecular Weight: | 291.07 |
| InChI: | InChI=1/Co.NO3.6H2O/c;2-1(3)4;;;;;;/h;;6*1H2/q+2;-1;;;;;; |
| Molecular Structure: |
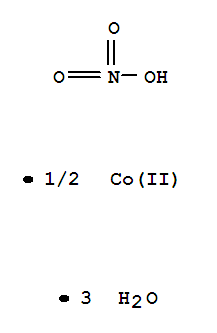 |
| Properties |
| Transport: | UN 1477 |
| Density: | 1.88 |
| Stability: | Stable. Strong oxidizer - incompatible with reducing agents. |
| Refractive index: | 1.52 |
| Water Solubility: | 134 G/100 ML |
| Solubility: | Water solubility: 134 g/100 mL |
| Appearance: | Red crystal, deliquescent in most air |
| Specification: |
?Cobaltous nitrate hexahydrate , its cas register number is 10026-22-9. It also can be called Cobalt(II) nitrate hexahydrate ; Cobalt dinitrate hexahydrate ; and Cobalt(2+) nitrate hexahydrate . The high solubility of cobalt nitrate makes it a common source of cobalt in metal-organic frameworks and polymers. It is also reduced to metallic cobalt or precipitated on various substrates for Fischer-Tropsch catalysis.
It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Wash mouth out with water, and get medical aid immediately.
In addition, Cobaltous nitrate hexahydrate (CAS NO.10026-22-9) could be stable under normal temperatures and pressures. It is not compatible with strong oxidizing agents, and you must not take it with incompatible materials, moisture, excess heat, combustible materials. And also prevent it to broken down into hazardous decomposition products:?Nitrogen oxides.
|
| Report: |
IARC Cancer Review: Group 2B IMEMDT ?? IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.:?) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT ?? IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.:?) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) .
|
| Packinggroup: | III |
| HS Code: | 28342920 |
| Storage Temperature: | Do not store near combustible materials. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Usage: | Commonly used in dyes and inks. |
| Safety Data |
| Hazard Symbols |
 O: Oxidizing agent
O: Oxidizing agent
 Xn: Harmful
Xn: Harmful
|
| |
 |