| Identification |
| Name: | 4-Bromophenyl phenyl ether |
| Synonyms: | 1-bromo-4-phenoxybenzene; 1-BROMO-4-PHENOXY-BENZENE (4-BROMOPHENYL PHENYL ETHER); 4-bromo-1-phenoxy-benzene; 4-Bromodiphenyl ether; p-Bromodiphenyl ether? 4-Bromophenoxybenzene; |
| CAS: | 101-55-3 |
| EINECS: | 202-952-4 |
| Molecular Formula: | C12H9BrO |
| Molecular Weight: | 249.11 |
| InChI: | InChI=1/C12H9BrO/c13-10-6-8-12(9-7-10)14-11-4-2-1-3-5-11/h1-9H |
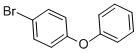
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 3082 |
| Density: | 1.423 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Refractive index: | n20/D 1.607(lit.) |
| Solubility: | Insoluble |
| Appearance: | colourless liquid |
| Specification: |
light yellow liquid
Safety Statements:60-61-62-36/37/39-26
60:This material and/or its container must be disposed of
as hazardous waste
61:Avoid release to the environment. Refer to special instructions
safety data sheet
62:If swallowed, do not induce vomiting: seek medical advice
immediately and show this container or label
36/37/39:Wear suitable protective clothing, gloves and eye/face
protection
26:In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice |
| Packinggroup: | III |
| Storage Temperature: | Store at away from heat, flames, or oxidizing materials. Ethers tend to oxidize when exposed to air, forming unstable peroxides that may detonate with extreme violence when concentrated by evaporation or distillation. When combined with other compounds that give a detonable mixture or when disturbed by heat, shock or friction. Appreciable quantities of crystalline solids or a viscous liquid in the bottom of ether-filled containers have been observed as gross evidence of formation of peroxides. |
| Usage: | Research chemical. |
| Safety Data |
| Hazard Symbols |
 N:Dangerousfortheenvironment
N:Dangerousfortheenvironment
|
| |
 |