| Identification |
| Name: | 1,2-Dimethoxyethane |
| Synonyms: | 1,2-ethanediol, dimethyl ether; 2,5-Dioxahexane; alpha,beta-dimethoxyethane; ansul ether 121; Dimethoxyethane; Dimethyl Cellosolve; DME; EGDME; ethylene dimethyl ether; Ethylene glycol dimethyl ether; GDME; Glycol dimethyl ether; Glyme; monoethylene glycol dimethyl ether; Monoglyme; MONOETHYLENGLYCOL; 1,2-dimethoxy-ethan; 1,2-Ethanediol dimethyl ether; 1,2-ethanediol,dimethylether; Ansol E-121; ansulether121; CH3OCH2CH2OCH3; dimethylcellosolvesolvent; Dimethylcollosolve; 1,2-dimethoxethane |
| CAS: | 110-71-4 |
| EINECS: | 203-794-9 |
| Molecular Formula: | C4H10O2 |
| Molecular Weight: | 90.12 |
| InChI: | InChI=1/C4H10O2/c1-5-3-4-6-2/h3-4H2,1-2H3 |
| Molecular Structure: |
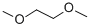 |
| Properties |
| Transport: | UN 2252 |
| Density: | 0.867 |
| Stability: | Stable, but explosive peroxides may form on exposure to air. Test for the presence of peroxides before heating. Avoid heat, light, air. Highly flammable. Store under inert gas. |
| Refractive index: | 1.3785-1.3805 |
| Water Solubility: | Miscible |
| Solubility: | Miscible with water |
| Appearance: | water-white Liquid |
| Specification: |
? 1,2-Dimethoxyethane , with CAS number of 110-71-4, can be called 1,2-Ethanediol, dimethyl ether ; DME ; Dimethyl Cellosolve ; Ethylene dimethyl ether ; Ethylene glycol dimethyl ether ; Glyme ; Monoethylene glycol dimethyl ether ; Monoglyme ; Ethylene glycol dimethy ether ; a,b-Dimethoxyethane . 1,2-Dimethoxyethane (CAS NO.110-71-4)? should be stored in a tightly closed container. Make sure to keep from contact with oxidizing materials. It must be stored in a cool, dry, well-ventilated area away from incompatible substances. Flammables-area.
|
| Report: |
Reported in EPA TSCA Inventory. Glycol ether compounds are on the Community Right-To-Know List.
|
| Packinggroup: | II |
| HS Code: | 29091900 |
| Sensitive: | Air Sensitive |
| Color: | WATER-WHITE LIQUID
COLORLESS LIQUID |
| Usage: | Solvent to facilitate formation of alkali metal-hydrocarbon adducts, in reformatsky reaction with methyl gamma-bromocrotonate. |
| Safety Data |
| Hazard Symbols |
 F:Flammable
F:Flammable
|
| |
 |