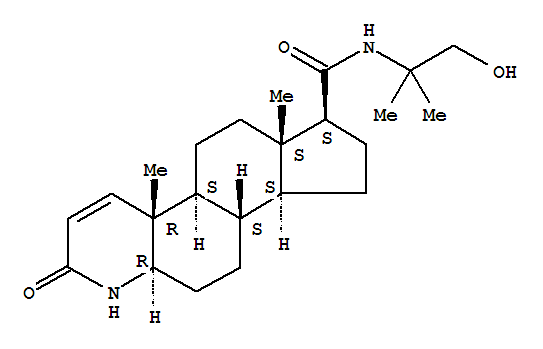
| Identification |
| Name: | 1H-Indeno[5,4-f]quinoline-7-carboxamide,2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-N-(2-hydroxy-1,1-dimethylethyl)-4a,6a-dimethyl-2-oxo-,(4aR,4bS,6aS,7S,9aS,9bS,11aR)- |
| Synonyms: | 4-Azaandrost-1-ene-17-carboxamide,N-(2-hydroxy-1,1-dimethylethyl)-3-oxo-, (5a,17b)-; 1H-Indeno[5,4-f]quinoline-7-carboxamide,1,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-N-(2-hydroxy-1,1-dimethylethyl)-4a,6a-dimethyl-2-oxo-,[4aR-(4aa,4bb,6aa,7a,9ab,9ba,11ab)]- |
| CAS: | 116285-36-0 |
| Molecular Formula: | C23H36 N2 O3 |
| Molecular Weight: | 388.54 |
| InChI: | InChI=1/C23H36N2O3/c1-21(2,13-26)25-20(28)17-7-6-15-14-5-8-18-23(4,12-10-19(27)24-18)16(14)9-11-22(15,17)3/h10,12,14-18,26H,5-9,11,13H2,1-4H3,(H,24,27)(H,25,28)/t14-,15?,16?,17+,18?,22-,23+/m0/s1 |
| Molecular Structure: |
 |
| Properties |
| Refractive index: | 1.537 |
| Usage: | A metabolite of Finasteride (M-1 metabolite), an inhibitor of 5a-reductase, the enzyme which converts testosterone to the more potent androgen, 5a-dihydrotestosterone |
| Safety Data |
| |
 |