| Identification |
| Name: | Zinc carbonate oxide (Zn3(CO3)O2), trihydrate (9CI) |
| Synonyms: | zinccarbonatehydroxidehydrate;CARBONIC ACID ZINC SALT;ZINC CARBONATE BASIC;ZINC SUBCARBONATE;ZINC OXIDE TRANSPARENCY;Zinc carbonate monohydrate;basic zinc carbonate;ZINC CARBONATE HYDROXIDE PURE (ZINC CARBONATE BASIC) |
| CAS: | 12539-71-8 |
| Molecular Formula: | CO3 . 3H2O . O . Zn |
| Molecular Weight: | 386.29 |
| InChI: | InChI=1/CH2O3.H2O.Zn/c2-1(3)4;;/h(H2,2,3,4);1H2;/q;;+2/p-2 |
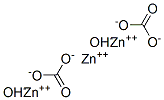
| Molecular Structure: |
 |
| Properties |
| Melting Point: | ca. 300 C (decomposes) |
| Flash Point: | °C |
| Boiling Point: | °Cat760mmHg |
| Density: | g/cm3 |
| Stability: | Stable. Hygroscopic. Incompatible with strong acids, strong oxidizing agents. |
| Appearance: | white powder |
| Flash Point: | °C |
| Safety Data |
| |
 |