| Identification |
| Name: | Mercury cyanide oxide(Hg2(CN)2O) |
| Synonyms: | Mercurycyanide oxide (Hg2O(CN)2) (8CI);Mercuric oxycyanide;Mercury oxycyanide;Mercury cyanide oxide;Dimercury dicyanide oxide;Mercury-II-oxycyanide;Mercury cyanide oxide (Hg2(CN)2O);Mercury(II) oxide cyanide;Dimercury dicyanide oxide; |
| CAS: | 1335-31-5 |
| EINECS: | 215-629-8 |
| Molecular Formula: | C2Hg2N2O |
| Molecular Weight: | 469.2142 |
| InChI: | InChI=1S/2CN.2Hg.O/c2*1-2;;; |
| Molecular Structure: |
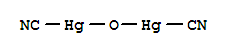 |
| Properties |
| Transport: | 1642 |
| Flash Point: | °C |
| Boiling Point: | °Cat760mmHg |
| Density: | g/cm3 |
| Water Solubility: | Slightly soluble in water |
| Solubility: | Slightly soluble in water |
| Specification: |
1.Reactivity Profile
Mercury cyanide oxide (1335-31-5) detonates on heating [Hawley]. Several instances have been reported where explosions occurred in handling this substance. Friction is a frequent cause of the explosion. Decomposes on contact with acid to give off hydrogen cyanide, a flammable poisonous gas. Desensitized by mixing 67% mercuric cyanide to create commercial product.
|
| Report: |
Mercury and its compounds, as well as cyanide and its compounds, are on the Community Right-To-Know List.
|
| Packinggroup: | II |
| Flash Point: | °C |
| Safety Data |
| Hazard Symbols |
 Detonates on heating, dangerous explosion risk. Highly toxic. TLV: TWA 0.1
Detonates on heating, dangerous explosion risk. Highly toxic. TLV: TWA 0.1
|
| |
 |