| Identification |
| Name: | Uranium, bis(nitrato-kO)dioxo-, hydrate (1:6), (T-4)-(9CI) |
| Synonyms: | Uranium,bis(nitrato-O)dioxo-, hexahydrate, (T-4)-;Uranium, dinitratodioxo-,hexahydrate (8CI);Dinitratodioxouranium hexahydrate;Uranium dioxidedinitrate, hexahydrate;Uranyl dinitrate hexahydrate;Uranyl nitratehexahydrate; |
| CAS: | 13520-83-7 |
| EINECS: | 233-266-3 |
| Molecular Formula: | H2O . 1/6 N2 O8 U |
| Molecular Weight: | 502.14 |
| InChI: | InChI=1/2HNO3.6H2O.2O.U/c2*2-1(3)4;;;;;;;;;/h2*(H,2,3,4);6*1H2;;;/r2HNO3.O2U.6H2O/c2*2-1(3)4;1-3-2;;;;;;/h2*(H,2,3,4);;6*1H2 |
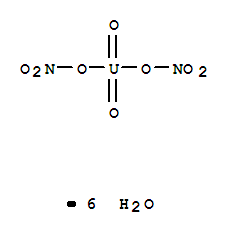
| Molecular Structure: |
 |
| Properties |
| Transport: | 2912 |
| Flash Point: | °C |
| Density: | 2,807 g/cm3 |
| Stability: | Radioactive, but chemically stable. Strong oxidant. Hygroscopic. Incompatible with combustible materials, reducing agents. May react explosively with cellulose and certain organic solvents. |
| Appearance: | Pale yellow crystalline solid |
| Specification: |
Acidic solution in water of uranyl nitrate, a radioactive yellow crystalline solid. Mildly chemically toxic. Contains nitric acid. Noncombustible, but will accelerate the burning of other combustible materials if concentrated or if the water evaporates. Large quantities may explode if exposed to fire. Produces toxic oxides of nitrogen if involved in fire. Radioactive materials emit certain rays which can be detected only by instruments.Dissolves in water forming a weak solution of nitric acid, the reaction is not hazardous.
|
| Packinggroup: | II |
| Flash Point: | °C |
| Safety Data |
| Hazard Symbols |
|
| |
 |