| Identification |
| Name: | Uranium,difluorodioxo-, (T-4)- |
| Synonyms: | Uranium,difluorodioxo- (8CI); Difluorodioxouranium; Uranium difluoride dioxide; Uraniumfluoride (238UO2F2); Uranium fluoride (UO2F2); Uranium fluoride oxide (UF2O2);Uranium fluoride oxide (UO2F2); Uranium oxide fluoride (UO2F2); Uraniumoxyfluoride; Uranium oxyfluoride (UO2F2); Uranyl fluoride; Uranyl fluoride(UO2F2); Uranyldifluoride |
| CAS: | 13536-84-0 |
| EINECS: | 236-898-8 |
| Molecular Formula: | F2O2 U |
| Molecular Weight: | 308.00 |
| InChI: | InChI=1/2FH.2O.U/h2*1H;;;/q;;;;+2/p-2/rF2O2U/c1-5(2,3)4 |
| Molecular Structure: |
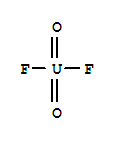 |
| Properties |
| Transport: | 2912 |
| Flash Point: | °C |
| Boiling Point: | °Cat760mmHg |
| Density: | g/cm3 |
| Solubility: | Insoluble in benzene
64.4 g/100 g H2O at 20 deg C |
| Specification: |
Uranyl fluoride ,its cas register number is 2445-07-0. It also can be called Uranium oxyfluoride ; Uranium, difluorodioxo-, (T-4)- ; and Uranyldifluoride . Uranyl fluoride (CAS NO.2445-07-0) is an intermediate in the conversion of uranium hexafluoride UF6 to an uranium oxide or metal form, and is reported to be stable in air to 300 °C. The reaction( UF6+ 2H2O → UO2F2+ 4HF) can take place whether it is a solid or a gas, but will take place almost instantaneously when the UF6 is in a gaseous state. Its chemical hazards are far more significant than radioactive hazards.
|
| Report: |
Reported in EPA TSCA Inventory.
|
| Flash Point: | °C |
| Color: | Pale yellow, rhombohedral, hygroscopic
Yellow, hygroscopic solid |
| Safety Data |
| |
 |