| Identification |
| Name: | Vanadium, ion (V1+) |
| Synonyms: | V1+;Vanadium ion(1+); Vanadium monocation; Vanadium(1+); Vanadium(1+) ion |
| CAS: | 14782-33-3 |
| Molecular Formula: | V |
| Molecular Weight: | 0 |
| InChI: | InChI=1S/V/q+1 |
| Molecular Structure: |
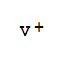 |
| Properties |
| Boiling Point: | 3407 deg C |
| Solubility: | Insoluble in water
Soluble in nitric, hydrofluoric, and concentrated sulfuric acids; attacked by alkali, forming water soluble vanadates |
| Color: | Light gray or white lustrous powder, fused hard lumps or body-centered cubic crystals
Pure vanadium is a bright white metal; soft and ductile
Gray-white metal; cubic.
Steel gray with a bluish tinge. |
| Safety Data |
| |
 |