| Identification |
| Name: | Silicate(2-),hexafluoro-, calcium (1:1) |
| Synonyms: | Calciumfluosilicate (6Cl);Calcium hexafluorosilicate (7Cl);Silicate(2-), hexafluoro-, calcium (1:1);Calcium fluorosilicate;Calcium silicofluoride;Hexafluorosilicate(2-) calcium (1:1); |
| CAS: | 16925-39-6 |
| EINECS: | 240-991-9 |
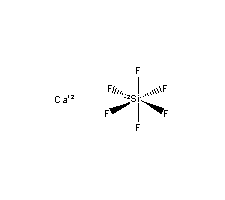
| Molecular Formula: | Ca.F6Si |
| Molecular Weight: | 182.16 |
| InChI: | InChI=1/Ca.6FH.Si/h;6*1H;/q+2;;;;;;;+4/p-6 |
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 2856 |
| Flash Point: | °C |
| Boiling Point: | °Cat760mmHg |
| Density: | g/cm3 |
| Solubility: | Soluble |
| Appearance: | Colorless tetragonal crystals |
| Specification: |
Calcium Silicofluoride (CAS NO.16925-39-6) is a colorless tetragonal crystals.
First Aid Measures:
Ingestion: Seek medical assistance.
Inhalation: Move victim to fresh air. Apply artificial respiration if victim is not breathing. Do not use mouth-to-mouth method if victim ingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a one-way valve or other proper respiratory medical device. Administer oxygen if breathing is difficult.
Skin: Remove and isolate contaminated clothing and shoes. Remove material from skin immediately. Immediately flush with running water for at least 20 minutes. For minor skin contact, avoid spreading material on unaffected skin.
Eyes: Immediately flush with running water for at least 20 minutes.
|
| Report: |
Reported in EPA TSCA Inventory.
|
| Flash Point: | °C |
| Usage: | Preservative in wood industry, hardener in concrete, opaliger in glass and porcelain enamel. Produce silicon tetrafluoride and calcium fluoride. . |
| Safety Data |
| |
 |