| Identification |
| Name: | Reserpine |
| Synonyms: | reserpine crystalline; 3,4,5-Trimethoxybenzoyl methyl reserpate; Reserpine,98%; Reserpine base; Resperine |
| CAS: | 50-55-5 |
| EINECS: | 200-047-9 |
| Molecular Formula: | C33H40N2O9 |
| Molecular Weight: | 608.68 |
| InChI: | InChI=1/C33H40N2O9/c1-38-19-7-8-20-21-9-10-35-16-18-13-27(44-32(36)17-11-25(39-2)30(41-4)26(12-17)40-3)31(42-5)28(33(37)43-6)22(18)15-24(35)29(21)34-23(20)14-19/h7-8,11-12,14,18,22,24,27-28,31,34H,9-10,13,15-16H2,1-6H3/t18-,22+,24-,27-,28+,31+/m1/s1 |
| Molecular Structure: |
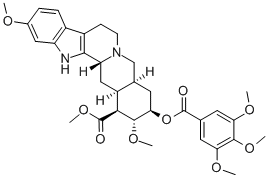 |
| Properties |
| Transport: | UN 1219 |
| Density: | 1.32g/cm3 |
| Stability: | Stable, but darkens slowly in light. Combustible. Incompatible with strong acids, reducing agents, oxidizing agents. |
| Water Solubility: | Insoluble |
| Solubility: | Insoluble |
| Appearance: | light yellow powder |
| Specification: |
off-white crystalline powder
usageEng:An indole alkaloid found in Rauwolfia serpentina. Inhibits vesicular uptake of catecholamines and serotonin. Reserpine is reasonably anticipated to be a human carcinogen. Antihypertensive.
Safety Statements:22-36/37/39-26
22:Do not breathe dust
36/37/39:Wear suitable protective clothing, gloves and eye/face
protection
26:In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice |
| Packinggroup: | II |
| Biological Activity: | Binds the vesicular monoamine transporter (VMAT2) and inhibits transport of biogenic amines into adrenal chromaffin granules and synaptic vesicles. Causes depletion of biogenic amine stores. Antihypertensive and antipsychotic. |
| Color: | White or pale buff to slightly yellowish powder
Long prisms from dil acetone |
| Usage: | An indole alkaloid found in Rauwolfia serpentina. Inhibits vesicular uptake of catecholamines and serotonin. Reserpine is reasonably anticipated to be a human carcinogen. Antihypertensive. |
| Safety Data |
| Hazard Symbols |
 Xi:Irritant
Xi:Irritant
|
| |
 |