| Identification |
| Name: | apigenin |
| Synonyms: | C.I. Natural Yellow 1;Prestwick_719;Versulin;4,5, 7-Trihydroxyflavone;2446-tetrahydroxychalcone;Apigenol;4H-1-Benzopyran-4-one,5,7-dihydroxy-2- (4-hydroxyphenyl)-;4H-1-Benzopyran-4-one, 5, 7-dihydroxy-2- (4-hydroxyphenyl)-;ND-9076;5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one;5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one;5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one;4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-;Spigenin;5,7,4-Trihydroxyflavone;4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (9CI);4,5,7-Trihydroxyflavone;5-18-04-00574 (Beilstein Handbook Reference);Chamomile;5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone;naringenin chalcone;Apigenine;2-(p-Hydroxyphenyl)-5,7-dihydroxychromone;Flavone, 4,5,7-trihydroxy-;Monomer Apigenin;Apigenin, 98% (HPLC); |
| CAS: | 520-36-5 |
| EINECS: | 208-292-3 |
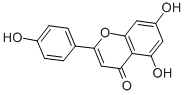
| Molecular Formula: | C15H10O5 |
| Molecular Weight: | 270.2369 |
| InChI: | InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H |
| Molecular Structure: |
 |
| Properties |
| Transport: | OTH |
| Density: | 1.548 g/cm3 |
| Refractive index: | 1.732 |
| Water Solubility: | DMSO: 27 mg/mL |
| Solubility: | DMSO: 27 mg/mL |
| Appearance: | Pale Yellow Crystalline Solid |
| Specification: |
? Apigenin , with CAS number of 520-36-5, can be called Apigenin, 98% (HPLC) ; 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone ; Flavone, 4,5,7-trihydroxy- ; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone ; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)- (9CI) ; 5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one ; 4H-1-Benzopyran-4-one,5,7-dihydroxy-2- (4-hydroxyphenyl)- . Apigenin (CAS NO.520-36-5) is a potent inhibitor of CYP2C9, an enzyme responsible for the metabolism of many pharmaceutical drugs in the body. Apigenin is a flavone that is the aglycone of several glycosides. Apigenin is a yellow crystalline solid that has been used to dye wool.
|
| HS Code: | 29329985 |
| Color: | yellow |
| Usage: | Induces the reversion of transformed phenotypes of v-H-ras-transformed NIH 3T3 cells at low concentration (12.5 uM) by inhibiting MAP kinase activity. Also inhibits the proliferation of malignant tumor cells by G2/M arrest and induces morphological diffe |
| Safety Data |
| Hazard Symbols |
 Xi:Irritant
Xi:Irritant
|
| |
 |