| Identification |
| Name: | Silver acetate |
| Synonyms: | Silver(I) acetate; Acetic acid silver(I) salt |
| CAS: | 563-63-3 |
| EINECS: | 209-254-9 |
| Molecular Formula: | C2H3AgO2 |
| Molecular Weight: | 166.91 |
| InChI: | InChI=1/C2H4O2.Ag/c1-2(3)4;/h1H3,(H,3,4);/q;+1/p-1 |
| Molecular Structure: |
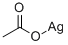 |
| Properties |
| Transport: | 1759 |
| Melting Point: | 2000 |
| Density: | 3.259 |
| Stability: | Stability Stable, but light sensitive. Incompatible with strong reducing agents. |
| Solubility: | 10.2 g/L (20 oC) |
| Appearance: | white crystalline powder |
| Specification: |
?Silver(I) acetate (CAS NO.563-63-3) is also named as NSC 112212 ; Silver acetate ;?Silver monoacetate ; Silver(1+) acetate ; UNII-19PPS85F9H?; Acetic acid, silver(1+) salt ; Acetic acid, silver(1+) salt (1:1)?.?Silver(I) acetate (CAS NO.563-63-3)?a photosensitive and white crystalline substance.?It is?light sensitive. It is slightly soluble in water. Silver(I) acetate is freely soluble in dilute nitric acid. It can serve as an oxidizing agent. The dust of Silver(I) acetate irritates nose and throat. Contact with eyes or skin causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of skin (argyria).
|
| Report: |
Reported in EPA TSCA Inventory.
|
| HS Code: | 28432900 |
| Storage Temperature: | Do not store in direct sunlight. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Usage: | Oxidizing agent for use in liquid ammonia: kline, kershner, inorg. |
| Safety Data |
| Hazard Symbols |
 Xi:Irritant
Xi:Irritant
|
| |
 |