| Identification |
| Name: | Sodium oxalate |
| Synonyms: | Disodium oxalate; Oxalic acid disodium salt; Ethanedioic acid sodium salt |
| CAS: | 62-76-0 |
| EINECS: | 200-550-3 |
| Molecular Formula: | Na2C2O4 |
| Molecular Weight: | 134 |
| InChI: | InChI=1/C2H2O4.2Na/c3-1(4)2(5)6;;/h(H,3,4)(H,5,6);;/q;2*+1/p-2 |
| Molecular Structure: |
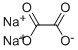 |
| Properties |
| Transport: | UN 2449/1759 |
| Flash Point: | 188.8 oC |
| Boiling Point: | 365.1oC at 760 mmHg |
| Density: | 2.34 |
| Stability: | Stability Stable; hygroscopic. Incompatible with strong oxidizing agents. |
| Solubility: | 37 g/L (20 oC) in water |
| Appearance: | White crystal or powder |
| Specification: |
?Sodium oxalate is a sodium salt of oxalic acid which can act as a reducing agent. Disodium oxalate is frequently referred to as sodium oxalate . The polyatomic ion of oxalate has a negative two charge, there can be no other compound consisting solely of sodium and oxalate other than disodium oxalate . Therefore, the prefix "di" is often dropped.
|
| Packinggroup: | III |
| HS Code: | 29171100 |
| Flash Point: | 188.8 oC |
| Storage Temperature: | Store at RT. |
| Sensitive: | Hygroscopic |
| Usage: | Finishing textiles, tanning and finishing leather. |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |