| Identification |
| Name: | 3,12-Epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-ol,decahydro-3,6,9-trimethyl-, (3R,5aS,6R,8aS,9R,10S,12R,12aR)- |
| Synonyms: | Cotecxin;DHQHS 2;Dihydroqinghaosu;Salaxin;Santecxin; |
| CAS: | 71939-50-9 |
| Molecular Formula: | C15H24O5 |
| Molecular Weight: | 284.35 |
| InChI: | InChI=1S/C15H24O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-13,16H,4-7H2,1-3H3/t8-,9-,10+,11+,12+,13-,14?,15-/m1/s1 |
| Molecular Structure: |
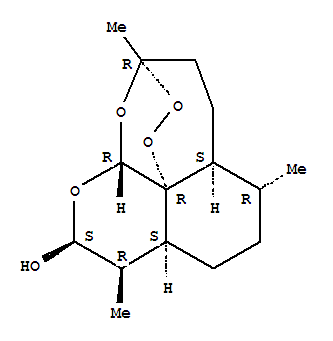 |
| Properties |
| Density: | 1.24 |
| Appearance: | White Solid |
| Specification: |
?Dihydroartemisinin (CAS No.:71939-50-9) is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. The lactone of artemisinin could selectively be reduced with mild hydride-reducing agents, such as sodium borohydride, potassium borohydride, and lithium borohydride to dihydroartemisinin (a lactol) in over 90% yield. It is a novel reduction, because normally lactone cannot be reduced with sodium borohydride under the same reaction conditions. Reduction with LiAlH4 leads to some rearranged products. It was surprising to find that the lactone was reduced, but that the peroxy group survived. However, the lactone of deoxyartemisinin resisted reduction with sodium borohydride and could only be reduced with isobutylaluminium hydride to the lactol, (deoxydihydroartimisinin). These results show that the peroxy group assists the reduction of lactone with sodium borohydride to a lactol, but not to the alcohol which is the over-reduction product. No clear evidence for this reduction process exists.
|
| Usage: | The main metabolite of Artemisinin, Arteether, Artemether, Artesunate. An active antimalarial metabolite |
| Safety Data |
| |
 |