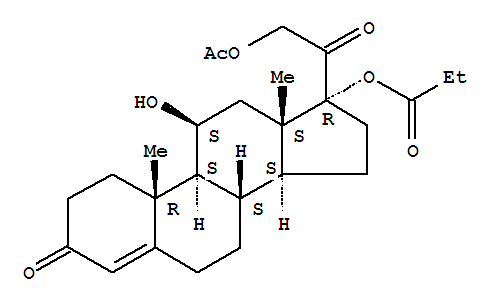
| Identification |
| Name: | Pregn-4-ene-3,20-dione,21-(acetyloxy)-11-hydroxy-17-(1-oxopropoxy)-, (11b)- |
| Synonyms: | Cortisol17-propionate 21-acetate; Hydrocortisone 17-propionate 21-acetate; Hydrocortisoneaceponate |
| CAS: | 74050-20-7 |
| Molecular Formula: | C26H36 O7 |
| Molecular Weight: | 0 |
| InChI: | InChI=1/C26H36O7/c1-5-22(31)33-26(21(30)14-32-15(2)27)11-9-19-18-7-6-16-12-17(28)8-10-24(16,3)23(18)20(29)13-25(19,26)4/h12,18-20,23,29H,5-11,13-14H2,1-4H3/t18-,19-,20-,23+,24-,25-,26-/m0/s1 |
| Molecular Structure: |
 |
| Properties |
| Flash Point: | 192.4°C |
| Boiling Point: | 589.3°C at 760 mmHg |
| Density: | 1.23g/cm3 |
| Refractive index: | 1.552 |
| Specification: |
White to Off-White Solid
usageEng:A new topical hydrocortisone derivative with esterification in positions 17 and 21, inhibit the incorporation of [3H]thymidine in DNA in human skin fibroblasts less strongly than the halogenated glucocorticosteroids betamethasone 17-valerate and clobetasol 17-propionate. Glucocorticosteroid |
| Flash Point: | 192.4°C |
| Storage Temperature: | Refrigerator |
| Usage: | A new topical hydrocortisone derivative with esterification in positions 17 and 21, inhibit the incorporation of [3H]thymidine in DNA in human skin fibroblasts less strongly than the halogenated glucocorticosteroids betamethasone 17-valerate and clobetasol 17-propionate. Glucocorticosteroid |
| Safety Data |
| |
 |