| Identification |
| Name: | Selenium sulfide (SeS2) |
| Synonyms: | Exsel;Seledruff;Seleen;Selenium disulfide;Selenium sulfide;Selsun; |
| CAS: | 7488-56-4 |
| EINECS: | 231-303-8 |
| Molecular Formula: | S2Se |
| Molecular Weight: | 143.092 |
| InChI: | InChI=1/S2Se/c1-3-2 |
| Molecular Structure: |
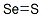 |
| Properties |
| Transport: | 2657 |
| Melting Point: | 100 °C
|
| Solubility: | Soluble in ammonium monosulfide; decomposes in aqueous nitric acid.
... Practically insoluble in water and organic solvents. |
| Specification: |
Fire Hazard of Selenium(IV) disulfide(1:2) (7488-56-4): Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
|
| Report: |
Selenium and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
|
| Packinggroup: | II |
| Color: | Brown red-yellow
Bright orange powder |
| Safety Data |
| Hazard Symbols |
 Toxic by ingestion, strong irritant to eyes and skin, an animal carcinogen. TLV: 0.2
Toxic by ingestion, strong irritant to eyes and skin, an animal carcinogen. TLV: 0.2
|
| |
 |