| Identification |
| Name: | Manganous chloride,anhydrous |
| Synonyms: | MANGANESE CHLORIDE; MANGANESE DICHLORIDE; MANGANESE(II) CHLORIDE; MANGANOUS CHLORIDE; di-manganesechlorid; Manganese bichloride; Manganese bister; Manganese chloride (MnCl2) |
| CAS: | 7773-01-5 |
| EINECS: | 231-869-6 |
| Molecular Formula: | MnCl2 |
| Molecular Weight: | 125.84 |
| InChI: | InChI=1/2ClH.Mn/h2*1H;/q;;+2/p-2 |
| Molecular Structure: |
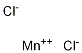 |
| Properties |
| Transport: | UN 3288 6.1/PG 3 |
| Stability: | Stable, but moisture sensitive. Incompatible with strong acids, reactive metals, hydrogen peroxide. |
| Solubility: | 723 g/L (25 oC) |
| Appearance: | pink powder |
| Specification: |
Manganese(II) chloride (7773-01-5) is rose red crystalline powder, also known as Manganese chloride ; Manganese chloride anhydrous ; Manganese bichloride ; Manganese dichloride ; Manganous chloride ; Scacchite ; Manganese chloride ; Manganese chloride (MnCl2) ; Manganese(II) chloride (1:2) . It can be made in many ways. It can be used as a contrast agent in MRI scanning. Manganese(II) chloride (7773-01-5) is incompatible with strong acids, reactive metals, hydrogen peroxide.
|
| Sensitive: | Hygroscopic |
| Color: | Pink trigonal crystals |
| Usage: | In dyeing (manganese bister), disinfecting, purifying natural gas, linseed oil drier, in electric batteries. |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |