| Identification |
| Name: | Hydrogen sulfide (H2S) |
| Synonyms: | Dihydrogenmonosulfide; Dihydrogen sulfide; Hydrogen sulfide; Hydrosulfuric acid; Stinkdamp; Sulfur dihydride; Sulfur hydride; Sulfur hydride (SH2); Sulfuretedhydrogen |
| CAS: | 7783-06-4 |
| EINECS: | 231-977-3 |
| Molecular Formula: | H2S |
| Molecular Weight: | 34.08 |
| InChI: | InChI=1/H2S/h1H2 |
| Molecular Structure: |
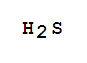 |
| Properties |
| Transport: | UN 1053 2.3 |
| Flash Point: | -82 C |
| Boiling Point: | -60 oC |
| Stability: | Stable. Highly flammable. May form explosive mixture with air. Note wide explosive limits. Incompatible with strong oxidizing agents, many metals. May react violently with metal oxides, copper, fluorine, sodium, ethanal. |
| Solubility: | INSOL IN WATER; SPARINGLY SOL IN ALC, ETHER
1 g/2 ml carbon disulfide
About 2.4% in benzene @ 30 deg C; more at higher temp
SOL IN TOLUENE
38.5% DISSOLVES IN LIQ AMMONIA (ANHYDR) @ -78 DEG C
2.65% DISSOLVES IN ACETONE @ 25 DEG C
9.1% DISSOLVES IN METHYLENE IODIDE @ 10 DEG C
ABOUT 1.5% DISSOLVES IN CHLOROFORM @ 18 DEG C
0.38% IN ANHYDR LANOLIN @ 45 DEG C (PRECIPITATED) /PHARMACEUTICAL/< |
| Appearance: | colourless gas with strong odour of rotten eggs |
| Packinggroup: | O52 |
| Flash Point: | -82 C |
| Color: | Alpha-sulfur, orthorhombic cyclooctasulfur. Amber-colored crystals. When heated becomes opaque owing to the formation of monoclinic sulfur.
Beta-sulfur, monoclinic cyclooctasulfur. Opaque, light-yellow, brittle, needle-like crystals.
Sublimed & washed sulfur are in form of fine, yellow crystalline powder /Pharmaceutical/
Precipitated sulfur is in form of very fine, pale yellow, amorphous or microcrystalline powder /Pharmaceutical/
Yellow solid or amber-colored molten liquid.
Pure sulfur exists in two stable crystalline forms, alpha and beta, and at least two amorphous (liquid) forms. Alpha-sulfur: rhombic, octahedral, yellow crystals; beta-sulfur: monoclinic, prismatic, pale-yellow crystals. |
| Safety Data |
| Hazard Symbols |
 T+: Very toxic
T+: Very toxic
 F+: Highly flammable
F+: Highly flammable
 N: Dangerous for the environment
N: Dangerous for the environment
|
| |
 |