| Identification |
| Name: | Selenic acid |
| Synonyms: | - |
| CAS: | 7783-08-6 |
| EINECS: | 231-979-4 |
| Molecular Formula: | H2O4Se |
| Molecular Weight: | 144.97 |
| InChI: | InChI=1/H2O4Se/c1-5(2,3)4/h(H2,1,2,3,4) |
| Molecular Structure: |
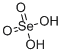 |
| Properties |
| Transport: | UN 3264 |
| Melting Point: | 50 C |
| Flash Point: | >230 °F |
| Boiling Point: | 260 C |
| Density: | 1.407 g/mL at 25 °C |
| Stability: | Stable, but decomposes on heating. Non-flammable. Incompatible with metals, combustible materials. Hygroscopic. |
| Refractive index: | n20/D 1.5174(lit.) |
| Solubility: | 1300 G SOL IN 100 ML WATER @ 30 DEG C
SOL IN SULFURIC ACID; INSOL IN AMMONIA |
| Appearance: | colourless or white crystals |
| Specification: |
Selenic acid is the chemical compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as (HO)2SeO2.
As predicted by VSEPR theory, the selenium center is tetrahedral, with a Se–O bond length of 161 pm. In the solid state, it crystallizes in an orthorhombic structure.
|
| Report: |
Selenium and its compounds are on the Community Right-To-Know List. EPA Genetic Toxicology Program. Reported in EPA TSCA Inventory.
|
| Packinggroup: | I |
| Flash Point: | >230 °F |
| Color: | White solid
Hexagonal prisms. |
| Safety Data |
| Hazard Symbols |
 T: Toxic
T: Toxic
|
| |
 |