| Identification |
| Name: | Sodium dichromate dihydrate (VI) |
| Synonyms: | Dichromic acid, (H2Cr2O7), disodium salt, dihydrate; Disodium dichromate dihydrate; sodium dichromate dihydrate; Sodium dichromate dihydrate (Na2Cr2O7.2H2O); |
| CAS: | 7789-12-0 |
| EINECS: | 234-190-3 |
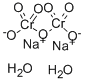
| Molecular Formula: | Na2Cr2O7?2(H2O) |
| Molecular Weight: | 298 |
| InChI: | InChI=1/2Cr.2Na.2H2O.7O/h;;;;2*1H2;;;;;;;/q;;2*+1;;;;;;;;2*-1/rCr2O7.2Na.2H2O/c3-1(4,5)9-2(6,7)8;;;;/h;;;2*1H2/q-2;2*+1;; |
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 3086 6.1/PG 1 |
| Flash Point: | 400 |
| Density: | 2.348 g/cm3 |
| Stability: | Stable under normal temperatures and pressures. |
| Refractive index: | 1.661 (17 C) |
| Solubility: | 730 g/L (20 oC) in water |
| Appearance: | orange crystals or needles |
| Specification: |
?Sodium dichromate dihydrate (CAS NO.7789-12-0) is also named as Disodium dichromate dihydrate ; Chromic acid (H2Cr2O7), disodium salt, dihydrate ; Chromic acid (H2Cr2O7), disodium salt, dihydrate (9CI) ; Dichromic acid (H2Cr2O7), disodium salt, dihydrate .?Sodium dichromate dihydrate (CAS NO.7789-12-0) is orange crystals or needles. It is?soluble in hot water. Sodium dichromate dihydrate can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). Reactions may be rapid but often requires initiation (heat, spark, catalyst, addition of a solvent). It can react violently with active metals, cyanides, esters, and thiocyanates.?Corrosive to mucous membranes continuous exposure may lead to perforation of nasal septum. Conjunctivitis and lacrimation to eyes. Corrosive producing deep penetrating ulcers to exposed area.?It has a harsh metallic taste.?Sodium dichromate dihydrate may cause vertigo, thirst, abdominal pain, vomiting, shock, convulsions and coma.
|
| Report: |
Chromium and its compounds are on the Community Right-To-Know List.
|
| Packinggroup: | III |
| HS Code: | 28413000 |
| Flash Point: | 400 |
| Storage Temperature: | Keep away from heat, sparks, and flame. Do not store near combustible materials. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Sensitive: | Hygroscopic |
| Color: | MONOCLINIC PRISMS
BRIGHT ORANGE RED
Red hydroscopic crystals |
| Usage: | Oxidizing agent in manufacture of dyes, many other synthetic organic chemicals, inks etc.; In chrome-tanning of hides; in electric batteries; bleaching fats, oils, sponges, resins; refining petroleum; manufacture chromic acid, other chromates and chrome pigments; in corrosion inhibitors, corrosion-inhibiting paints; in many metal treatments; electroengraving of copper; mordant in dyeing. |
| Safety Data |
| Hazard Symbols |
 O:Oxidizingagent
O:Oxidizingagent
 T+:Verytoxic
T+:Verytoxic
 N:Dangerousfortheenvironment
N:Dangerousfortheenvironment
|
| |
 |