| Identification |
| Name: | Chloric acid |
| Synonyms: | Chloricacid (HClO3) |
| CAS: | 7790-93-4 |
| EINECS: | 232-233-0 |
| Molecular Formula: | ClH O3 |
| Molecular Weight: | 84.46 |
| InChI: | InChI=1/ClHO3/c2-1(3)4/h(H,2,3,4) |
| Molecular Structure: |
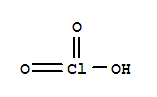 |
| Properties |
| Transport: | 2626 |
| Boiling Point: | >100 °C |
| Density: | 1.2 g/mL at 25 °C |
| Solubility: | 8.61 g/100 g water at 25 deg C
1 g dissolves slowly in 16.5 ml water, 1.8 ml boiling water, about 50 ml glycerol; almost insol in alcohol
SLIGHTLY SOL IN LIQUID AMMONIA; INSOL IN ACETONE; SOL IN ALKALIES
In water, 70,000 mg/L at 25 deg C [Shiu WY et al; Rev Environ Contam Toxicol 116: 15-187 (1990)] PubMed Abstract |
| Specification: |
Safety Statements:17-26-36/37/39-45
17:Keep away from combustible material
26:In case of contact with eyes, rinse immediately with plenty
of water and seek medical advice
36/37/39:Wear suitable protective clothing, gloves and eye/face
protection
45:In case of accident or if you feel unwell, seek medical
advice immediately (show label where possible) |
| Packinggroup: | II |
| Color: | Colorless, lustrous crystals or white granules or powder
White monoclinic crystals |
| Safety Data |
| Hazard Symbols |
|
| |
 |