| Identification |
| Name: | Ferrous gluconate dihydrate |
| Synonyms: | D-GLUCONIC ACID, IRON(II) SALT DIHYDRATE;GLUCONIC ACID IRON(II) SALT DIHYDRATE;GLUCONIC ACID IRON(II) SALT HYDRATE;FERROUS GLUCONATE DIHYDRATE;IRON(II) D-GLUCONATE DIHYDRATE;IRON (II) GLUCONATE;IRON(II) GLUCONATE DIHYDRATE;IRON(II) GLUCONATE HYDRATE |
| CAS: | 12389-15-0 |
| Molecular Formula: | C12H22FeO14.2(H2O) |
| Molecular Weight: | 482.17 |
| InChI: | InChI=1/2C6H12O7.Fe.2H2O/c2*7-1-2(8)3(9)4(10)5(11)6(12)13;;;/h2*2-5,7-11H,1H2,(H,12,13);;2*1H2/q;;+2;;/p-2/t2*2-,3-,4+,5-;;;/m11.../s1 |
| Molecular Structure: |
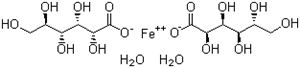 |
| Properties |
| Flash Point: | 673.6 oC at 760 mmHg |
| Boiling Point: | 673.6 oC at 760 mmHg |
| Solubility: | Soluble in glycerin
SOLUBILITY INCR BY ADDN OF CITRIC ACID OR CITRATE ION
1 gram dissolves in about 10 ml of water with slight heating and in 1.3 ml of water at 100 deg C. It forms supersaturated solutions which are stable for a period of time. |
| Flash Point: | 673.6 oC at 760 mmHg |
| Color: | The color of /ferrous gluconate/ solution depends on pH; they are light yellow at pH 2, brown at pH 4.5, and green at pH 7. The iron rapidly oxidizes at higher pH. |
| Safety Data |
| |
 |