| Identification |
| Name: | Aluminum nitrate |
| Synonyms: | Aluminum trinitrate;aluminum salt;Nitric acid,compounds,aluminum salt;Nitric acid, alumium salt;see Peroxide,nitro 1-oxopropyl;Nitric acid, aluminum salt (8CI 9CI);Aluminum(III) nitrate (1:3); |
| CAS: | 13473-90-0 |
| EINECS: | 236-751-8 |
| Molecular Formula: | Al.(NO3)3 |
| Molecular Weight: | 213 |
| InChI: | InChI=1/Al.3NO3/c;3*2-1(3)4/q+3;3*-1 |
| Molecular Structure: |
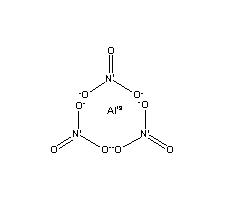 |
| Properties |
| Transport: | UN 1438 |
| Boiling Point: | 83 oC at 760 mmHg |
| Density: | 1.25 |
| Stability: | Stable under normal temperatures and pressures. |
| Solubility: | 640 g/L |
| Appearance: | white, crystalline solid |
| Specification: |
? Aluminum(III) nitrate (1:3) (CAS NO.13473-90-0), its Synonyms are Aluminum nitrate ; Aluminum trinitrate ; Nitrato de aluminio ; Nitric acid, aluminum salt (3:1) ; Nitric acid, aluminum salt ; Nitric acid, aluminum(3+) salt ; Nitric acid, alumium salt .
|
| Report: |
Reported in EPA TSCA Inventory.
|
| Packinggroup: | III |
| Storage Temperature: | Do not store near combustible materials. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep away from flammable liquids. |
| Usage: | Tanning leather, antiperspirant, corrosion inhibitor, extraction of uranium, nitrating agent. |
| Safety Data |
| Hazard Symbols |
 Xi:Irritant
Xi:Irritant
|
| |
 |