| Identification |
| Name: | Aluminum nitrate nonahydrate, low mercury |
| Synonyms: | Nitricacid, aluminum salt, nonahydrate (8CI,9CI); Aluminum nitrate nonahydrate;Aluminum trinitrate nonahydrate; Aluminum(III) nitrate nonahydrate |
| CAS: | 7784-27-2 |
| EINECS: | 236-751-8 |
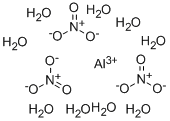
| Molecular Formula: | Al(NO3)3?9(H2O) |
| Molecular Weight: | 375.13 |
| InChI: | InChI=1/Al.3NO3.9H2O/c;3*2-1(3)4;;;;;;;;;/h;;;;9*1H2/q+3;3*-1;;;;;;;;; |
| Molecular Structure: |
 |
| Properties |
| Transport: | UN 1438 |
| Flash Point: | 135 |
| Density: | 1.25 |
| Stability: | Strong oxidizer - contact with combustible material may lead to fire. Incompatible with water, most common metals, organics. Moisture-sensitive. |
| Refractive index: | 1.54 |
| Solubility: | 64 g/100 mL (25 oC) |
| Appearance: | white crystalline powder |
| Packinggroup: | III |
| HS Code: | 28342980 |
| Flash Point: | 135 |
| Storage Temperature: | Keep away from heat, sparks, and flame. Do not store near combustible materials. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Keep away from flammable liquids. Store protected from moisture. |
| Sensitive: | Hygroscopic |
| Usage: | Aluminum nitrate nonahydrate is used in the preparation of insulating papers, on transformer core laminates and in cathode-ray tube heating elements; as a tanning agent, antiperspirant, nitrating agent, corrosion inhibitor, catalyst in petroleum refining and in uranium extraction; and in the manufacture of incandescent filaments. |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |