| Identification |
| Name: | 2-Nitrophenylacetonitrile |
| Synonyms: | 2-Nitrobenzyl cyanide; 2-nitrobenzeneacetonitrile; o-nitrobenzyl cyanide; 2-nitrophenyl acetic acid, nitrile; o-nitrophenylacetonitrile; |
| CAS: | 610-66-2 |
| EINECS: | 210-231-0 |
| Molecular Formula: | C8H6N2O2 |
| Molecular Weight: | 162.15 |
| InChI: | InChI=1/C8H6N2O2/c9-6-5-7-3-1-2-4-8(7)10(11)12/h1-4H,5H2 |
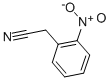
| Molecular Structure: |
 |
| Properties |
| Transport: | 3439 |
| Density: | 1.272 g/cm3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| Refractive index: | 1.577 |
| Water Solubility: | | <0.01 g/100 mL at 20 ºC |
| Solubility: | <0.01 g/100 mL at 20 ºC |
| Appearance: | light brown solid |
| Specification: |
Reactivity Profile of 2-NITROBENZYL NITRILE (610-66-2):
Reactivity Profile Nitriles, such as 2-Nitrophenylacetonitrile , may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids
|
| Report: |
Reported in EPA TSCA Inventory.
|
| Packinggroup: | III |
| Storage Temperature: | Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. |
| Safety Data |
| Hazard Symbols |
 Xn:Harmful
Xn:Harmful
|
| |
 |